Biography
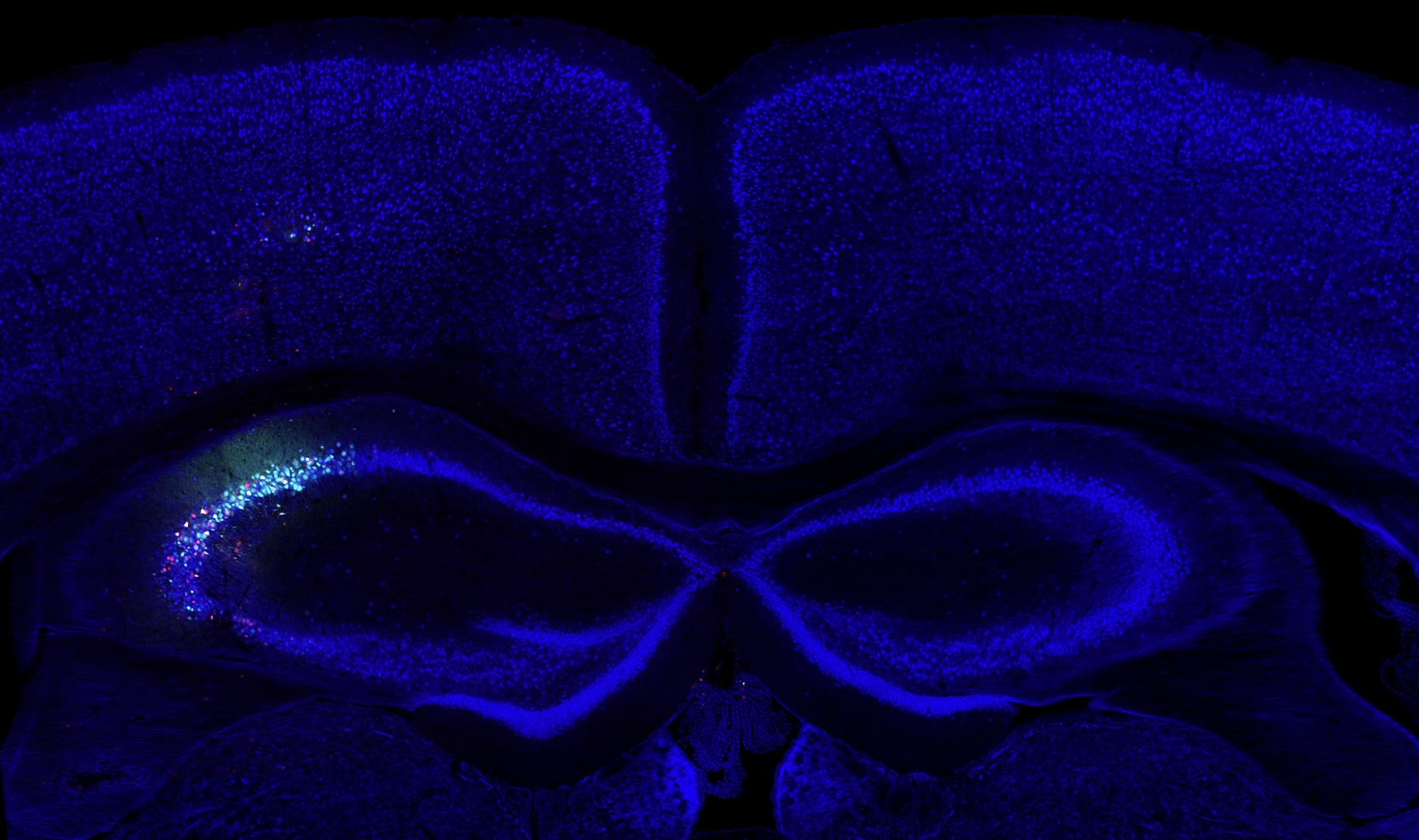
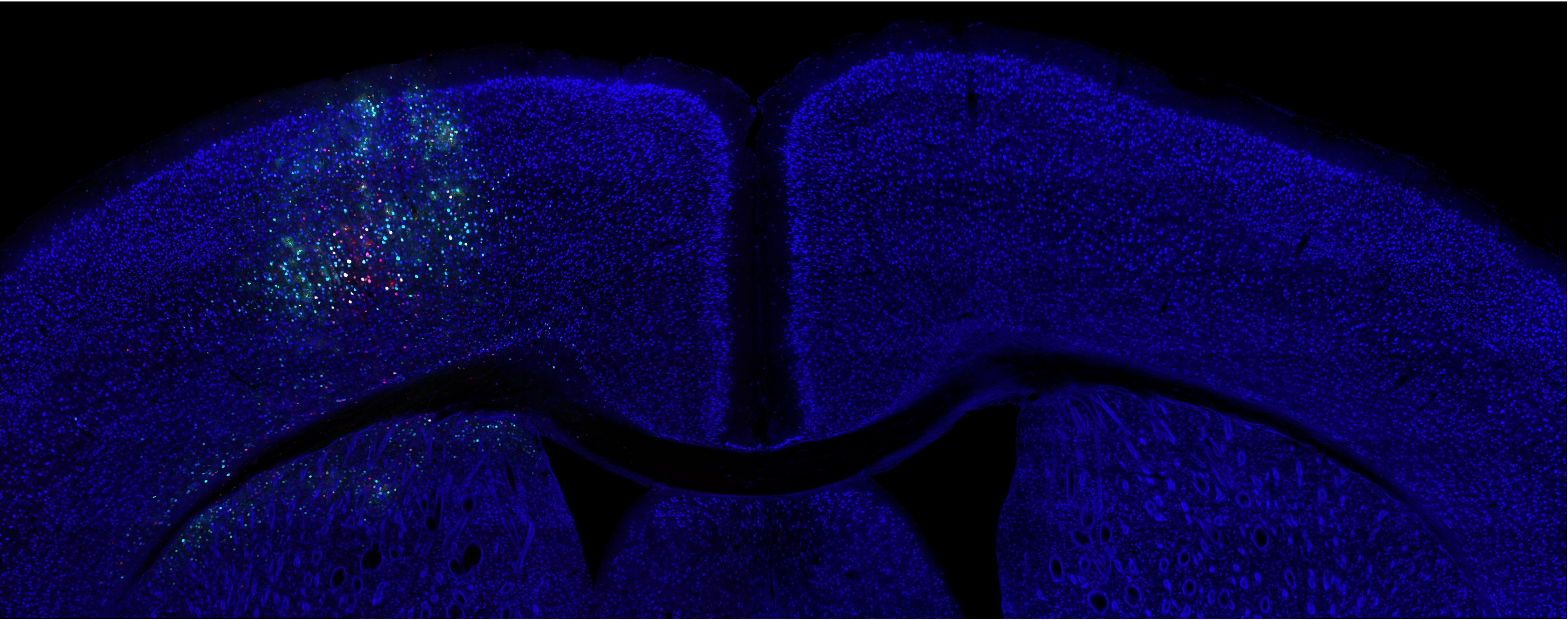
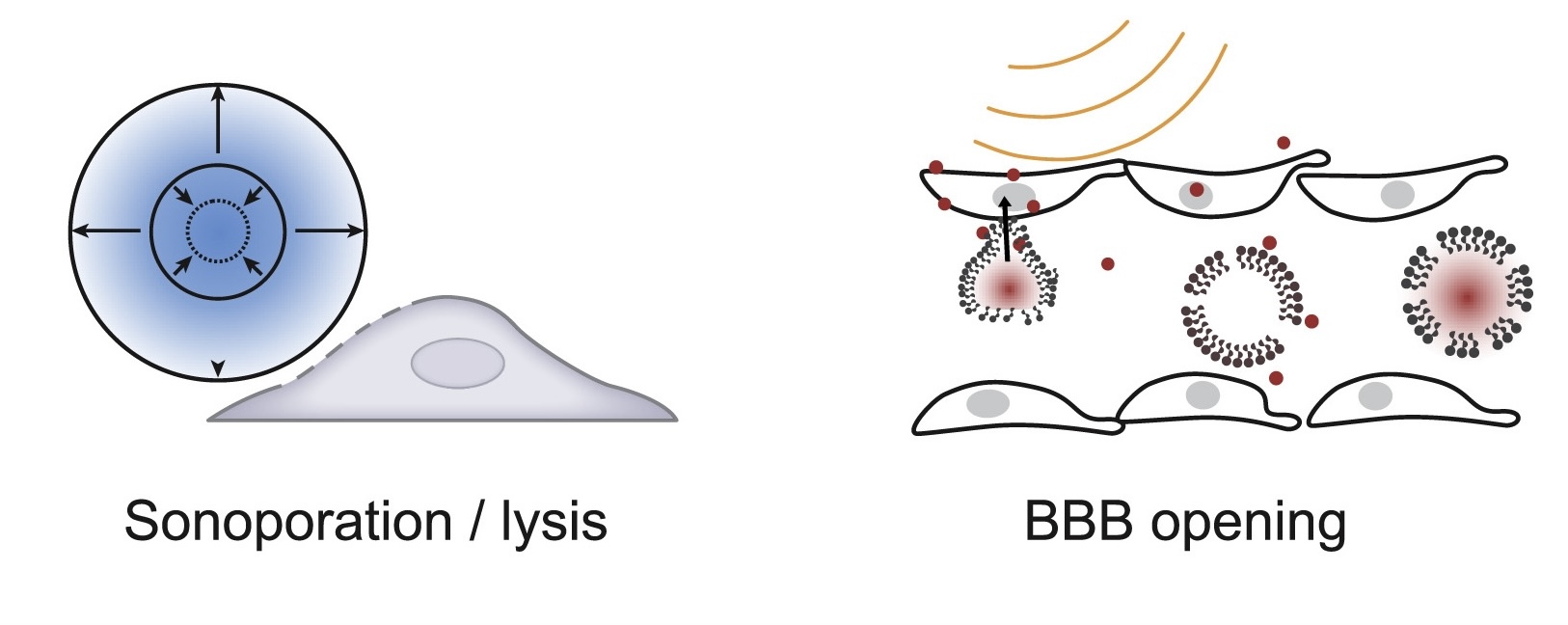
I am a postdoctoral associate at MIT co-advised by Katie Galloway and Christopher Voigt. My current research centers on developing delivery technologies to achieve scalable, targeted, and efficient human genome engineering. My PhD work focused on engineering acoustically targeted gene delivery that enables ultrasound mediated non-invasive, site-specific, cell type-specific transgene delivery to the brain in various animal models. My long-term research goal is to build interfacing platforms for non-invasive surveillance and modulation of brain-wide neural activities through genetic engineering and biomolecular ultrasound. Beyond molecular brain-machine interface, I am fascinated in tackling degenerative diseases, and eventually achieving organismal longevity, through fine tuning or reprogramming human cell state using human genome editing tools we developed.
- Neuroengineering
- Synthetic Biology
- Biomolecular Ultrasound
- Material Science
- Tissue Engineering
-
PhD in Biology & Biological Engineering, 2025
California Institute of Technology
-
BSc in Biomedical Engineering, 2017
Washington University in St. Louis
Experience
Contact
- li.richard.biomed@gmail.com
- (978) 518-5426
- 25 Ames St, Cambridge, MA 02139